Orthomolecular and Nutritional Consulting Toronto
My Blog & Health Articles
The Gut Brain Axis: Part 2
The Gut Brain Axis: Part 2
by Lara Rodin RNCP / ROHP, DSHomMed, FCHM
This is Part 2 of my Gut Brain Axis article, a dive into a medical research review article I found entitled “Gut microbiota’s effect on mental health: The gut-brain axis.” which I will refer to as the “review.”
In Part 1 of this series, I looked at 5 ways that the gut and brain communicate:
· Gut to Brain Communication #1: Hormones like Serotonin
· Gut to Brain Communication #2: The Vagus Nerve
· Gut to Brain Communication #3: The Microbiota-Gut-Brain-Axis
· Gut to Brain Communication #4: Neurotransmitters like GABA
· Gut to Brain Communication #5: Immunological Factors
Let’s continue this discussion and look closer at the immunological factors in gut brain communication, how dysbiosis and inflammation affect this communication, and finally how the brain communicates with the gut.
Dysbiosis
When we speak of the gut microbiota, we are talking about the more than 100 trillion microorganisms that inhabit the human gastrointestinal tract. This includes several species of microorganisms, including bacteria, yeast and viruses.1
Each person’s gut microbiota has been shaped in early life by factors such as type of delivery (C-section vs vaginal birth) and methods of feeding (bottle fed vs breast-feed). And it continues to be influenced throughout life by factors such as age, lifestyle, diet and antibiotic use.2
While there is no “one size fits all perfect” microbiota composition (since it is different for each individual), nevertheless a healthy balance between host and micro-organisms must be maintained to allow the gut to perform its metabolic and immune functions.3
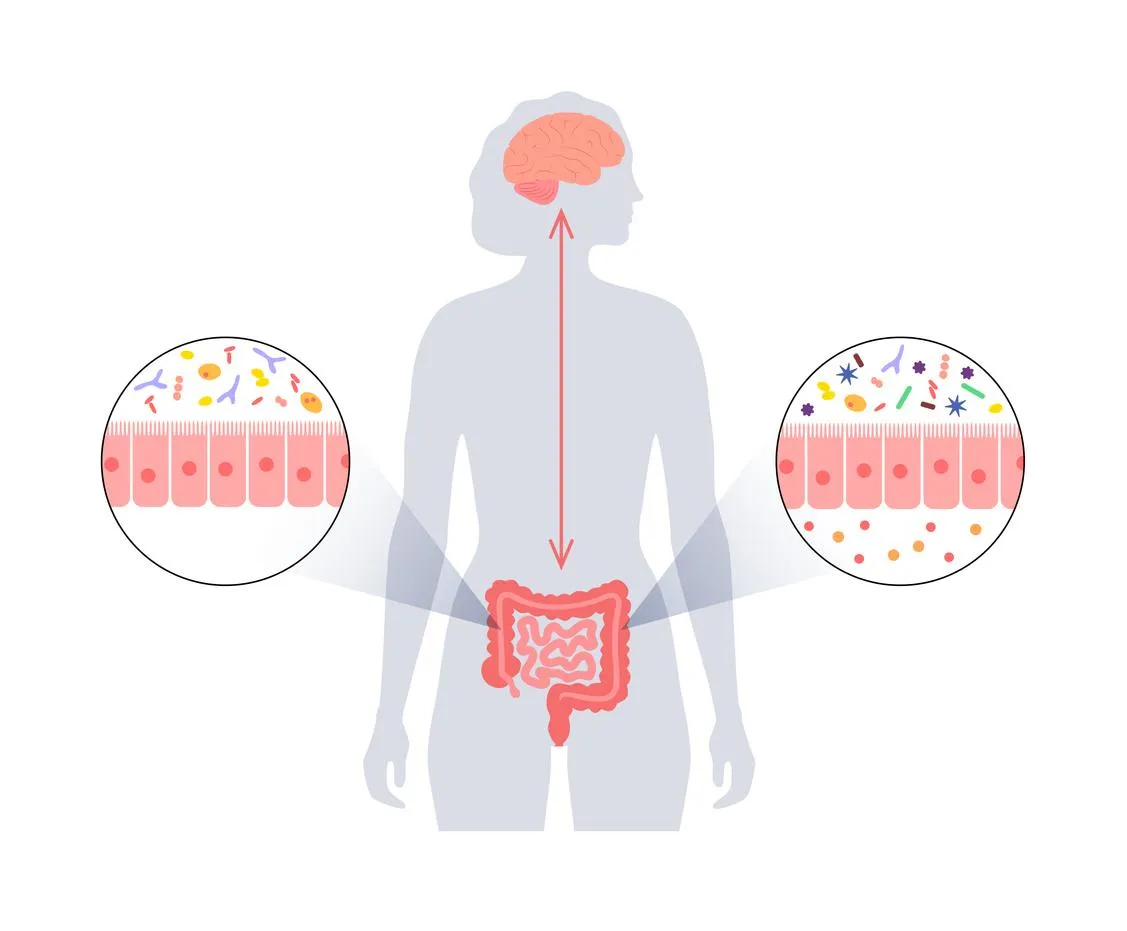
When the gut microbiota encounters change in diet, stress or antibiotics, the makeup of this normal flora undergoes changes which can disrupt this delicate balance and cause a condition called dysbiosis.4
Simply put, dysbiosis is when the balance of “good” bacteria to “bad” bacteria changes in favour of the bad or pathogenic bacteria. This leads to a break down in the integrity of the intestinal wall and leads to increased intestinal permeability, aptly called “leaky gut”. This can allow bacterial metabolites and products, as well as harmful bacteria themselves, to leak through the intestinal wall and into systemic circulation.5
The review neatly sums up the problem associated with having a leaky gut by summarizing:
“Increased intestinal permeability leads to detrimental effects on the host immune system, which have been demonstrated in diseases such as inflammatory bowel disease (IBD), diabetes, asthma, and psychiatric disorders including depression, anxiety, and autism.6 (Clapp et al., 2017, p. 132)
This is how a seemingly localized gut issue such as dysbiosis can affect whole body health; via increased intestinal permeability that leads to immune system activation and inflammation.
Dysbiosis, Leaky Gut and LPS
When discussing bacteria, it is often helpful to divide them into gram-negative and gram-positive. This is based on the research Christian Gram did over 100 years ago when he developed a staining procedure that allowed him to classify almost all bacteria into two groups; one that retained the stain (Gram-positive) and the other that did not (Gram-negative).7
For example, the bacteria e. coli (the pathogenic strains of which can cause food poisoning) is gram-negative.8
Why do you care? The issue is that these gram-negative bacteria have an outer membrane containing lipopolysaccharide or LPS, and when these bacteria die, the LPS gets released.9
Remember, these organisms live within our gastrointestinal tract, so when they die, they die in our intestines and their cell contents get spilled into our intestinal tract when their cell walls break open. If we have a leaky gut then those cell contents can be absorbed through the intestinal wall into systemic circulation.
Our immune system cells detect LPS and signals the body to start cytokine production, making such immune factors such as tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6) and interleukin-1 (IL-1).10
The problem is that it is believed this inflammatory response may be associated with depression.11
Dysbiosis and Inflammation
So, to recap, dysbiosis can lead to a leaky gut, allowing harmful microbiota derived products to enter systemic circulation and create inflammation in the body and this is an immune response which can be linked to depression.
The review sums this up nicely by saying: “Dysbiosis and inflammation of the gut have been linked to causing several mental illnesses including anxiety and depression, which are prevalent in society today.12 (Clapp et al., 2017, p. 131)
But it gets worse, because the elevated levels of cytokines, such as TNF- α, also increase the permeability of the Blood Brain Barrier13,14 which is a protective layer that acts like a gatekeeper for your brain and keeps harmful things out.15
And that brings us to back to the topic of inflammation and the fifth way the gut can communicate with the brain, via immunological factors and the immune system.
Dysbiosis, Inflammation and the Blood Brain Barrier
As discussed dysbiosis, when coupled with leaky gut, allows harmful microbiota derived molecules (endotoxins like LPS) to travel systemically in the blood. This activates the immune system and so the body produces inflammatory cytokines, such as TNF- α. While TNF- α is a critical part of a normal immune response, excessive production of TNF- α can be harmful and may lead to gut conditions such as irritable bowel disease (IBD) and skin conditions such as psoriasis.16
But this immune response can also affect our mental wellbeing, as elevated blood levels of cytokines such as TNF- α increase the permeability of blood brain barrier, and this can influence brain function, leading to anxiety, depression and memory loss. 17,18,19
In fact, one study showed a direct correlation between increased levels of Il-6 and TNF- α with symptoms of depression and anxiety. 20 The study gave endotoxin infusions to healthy subjects with no history of depression, and the ensuing cytokine release seemed to trigger classical depressive symptoms. However, correlation may not mean causation and the mood disturbance may be due to how the study itself was constructed using endotoxins, indicating we need to understand more about the inflammation / mood disturbance link. But there is yet another issue, because these pro-inflammatory cytokines also stimulate the stress response via the HPA axis.21
What is the HPA Axis?
When our CNS perceives stress, it activates the sympathetic nervous system and kicks in the fight our flight response, and it does so via the Hypothalamus Pituitary Adrenal (HPA) Axis. Referring to the “HPA Axis” is a shorter way to say that the hypothalamus stimulates the anterior pituitary which stimulates the adrenal glands to give us the stress response.
Our adrenal glands, little glands that sit atop of the kidneys, produce 3 main the hormones in the stress response: epinephrine (also known as adrenaline), norepinephrine and cortisol. These hormones are designed to help use deal with stressful situation giving us the ability to flee or fight by increasing our heart rate, blood pressure and blood sugar levels.22
Simply put, the stress response is when your HPA axis tells the body to release stress hormones in response to your brain perceiving stress. And this is great; you can run away from the bear or fight off the wolves at your door!
But if this response is constantly over-stimulated it can have negative effects on our bodies and minds. Unfortunately, our hectic modern lifestyles can trigger this system, as can those pro-inflammatory cytokines that we discussed earlier in relation to dysbiosis and leaky gut. And that can be a negative double-punch for the gut microbiota.
And this brings us to how our brain can affect our gut, via the HPA Axis.
Brain to Gut #1: Stress Stimulates the HPA Axis
We all recognized the concept that ongoing stress is our lives is bad … but exactly how does stress, something that seemingly only affects our brains, affect our gut? We have seen that stress and circulating pro-inflammatory cytokines can activate the stress response via the HPA Axis – but how exactly does this affect our gut?
The “by what mechanism” is that the stress response can lead to change in the amount and type of bacteria in our gut, which can alter the balance of bacteria and create dysbiosis.23
We discussed dysbiosis earlier, but many of you may be more familiar of the term in relation to what happens when someone takes antibiotics. In acute illnesses, antibiotics are prescribed to kill the “bad” bacteria that is causing an infection. But it may also kill off a lot of the protective bacteria in the gut and thus can lead to dysbiosis. This is why, for example, women are often more susceptible to a yeast infection after a course of antibiotics; the antibiotics can affect the delicate balance of the vaginal microbiota and leave it vulnerable to the yeast species that normally co-exist but are kept in check by protective bacteria. These yeast species then proliferate and create a yeast infection!24
With stress, instead of the stimulus being antibiotic, the stress response stimulates the release of various signaling molecules which can alter the composition of the gut microbiota. For example, a study showed that norepinephrine, one of hormones released after stress, can stimulate the increases of bad bacteria in the gut.25
An over abundance of bad or pathogenic bacteria, disturbs the balance of the organisms in the gut microbiota and thus affects health of the gastrointestinal tract. As mentioned, this can cause a breakdown of the integrity of the gut barrier and which leads to an increase in gut permeability (aka leaky gut syndrome) and, as we have discussed, that is a problem!
Good news is that with nutritional strategies and lifestyle adjustments, there is a lot we can do to support the health of our gut brain axis.
For gut to brain communication, probiotics, prebiotics and fermented food can help support the gut microbiota, and thus indirectly support mental wellbeing. Please read my next blog: “Gut Brain Axis: the role of Probiotics, Prebiotics and Fermented Foods.”
For brain to gut communication, calming the stress response and working with the Vagus nerve will help keep the effects of stress to a minimum and thus help protect the gut microbiota. Watch for my blog entitled “Gut Brain Axis: the Vagus Nerve.”
Conclusion:
The Gut-Brain-Axis (really the Microbiota-Gut-Brain-Axis) is a complex system of communication between the gut microbiota, the intestinal tract (including the enteric nervous system, gut hormones and the EECs) and your CNS.
The gut communicates with the CNS directly via the Vagus nerve, and indirectly via hormones, neurotransmitters and the immune system.
Dysbiosis and inflammation play a big role here in how they affect the gut, and that, in turn, affects the rest of the body and brain, as dysbiosis and inflammation in the CNS have now been linked as potential causes of mental illness.26
The brain / CNS affects the gut via the HPA axis and the stress response.
Understanding these connections offers ways for us, as busy women leading stressful lives, to help offset the damaging effects of stress on our bodies, thus protecting our gut microbiota and reducing the chances of negative cross talk between our gut and our brains, to support our mental wellbeing.
DISCLAIMER
The content provided by Lara Rodin in the Inside Out Microbiome Makeover and / or Live Well With Lara website, social media and other public communications, is for general informational and educational purposes only. It is not intended to diagnose, treat, cure or prevent any health condition or disease. It may not be suitable for your individual needs. You agree to use the content at your own discretion and risk.
Please consult with your personal healthcare provider before following any of the information or suggestions contained in the Inside Out Microbiome Makeover and /or Live Well With Lara website, social media and other public communications, including changes to your diet, exercise, medications, supplements, or other health and lifestyle practices.
REFERNCES AND WORKS CITED
Rinninella E, Raoul P, Cintoni M, et al. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms. 2019;7(1):14. Published 2019 Jan 10. doi:10.3390/microorganisms7010014
Rinninella E, Raoul P, Cintoni M, et al. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms. 2019;7(1):14. Published 2019 Jan 10. doi:10.3390/microorganisms7010014
Rinninella E, Raoul P, Cintoni M, et al. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms. 2019;7(1):14. Published 2019 Jan 10. doi:10.3390/microorganisms7010014
Clapp M, Aurora N, Herrera L, Bhatia M, Wilen E, Wakefield S. Gut microbiota's effect on mental health: The gut-brain axis. Clin Pract. 2017 Sep 15;7(4):987. doi: 10.4081/cp.2017.987. PMID: 29071061; PMCID: PMC5641835.
Clapp M, Aurora N, Herrera L, Bhatia M, Wilen E, Wakefield S. Gut microbiota's effect on mental health: The gut-brain axis. Clin Pract. 2017 Sep 15;7(4):987. doi: 10.4081/cp.2017.987. PMID: 29071061; PMCID: PMC5641835.
Clapp M, Aurora N, Herrera L, Bhatia M, Wilen E, Wakefield S. Gut microbiota's effect on mental health: The gut-brain axis. Clin Pract. 2017 Sep 15;7(4):987. doi: 10.4081/cp.2017.987. PMID: 29071061; PMCID: PMC5641835.
Silhavy TJ, Kahne D, Walker S. The bacterial cell envelope. Cold Spring Harb Perspect Biol. 2010 May;2(5):a000414. doi: 10.1101/cshperspect.a000414. Epub 2010 Apr 14. PMID: 20452953; PMCID: PMC2857177.
Lim JY, Yoon J, Hovde CJ. A brief overview of Escherichia coli O157:H7 and its plasmid O157. J Microbiol Biotechnol. 2010 Jan;20(1):5-14. PMID: 20134227; PMCID: PMC3645889.
Sun LJ, Li JN, Nie YZ. Gut hormones in microbiota-gut-brain cross-talk. Chin Med J (Engl). 2020 Apr 5;133(7):826-833. doi: 10.1097/CM9.0000000000000706. PMID: 32132364; PMCID: PMC7147657.
Sun LJ, Li JN, Nie YZ. Gut hormones in microbiota-gut-brain cross-talk. Chin Med J (Engl). 2020 Apr 5;133(7):826-833. doi: 10.1097/CM9.0000000000000706. PMID: 32132364; PMCID: PMC7147657.
Wright CE, Strike PC, Brydon L, Steptoe A. Acute inflammation and negative mood: mediation by cytokine activation. Brain Behav Immun 2005; 19:345–350. doi: 10.1016/j.bbi.2004.10.003. [PubMed] [Google Scholar]
Clapp M, Aurora N, Herrera L, Bhatia M, Wilen E, Wakefield S. Gut microbiota's effect on mental health: The gut-brain axis. Clin Pract. 2017 Sep 15;7(4):987. doi: 10.4081/cp.2017.987. PMID: 29071061; PMCID: PMC5641835
Biesmans S, Bouwknecht JA, Ver Donck L, et al. Peripheral administration of tumor necrosis factor-alpha induces neuroinflammation and sickness but not depressive-like behavior in mice. BioMed Res Int 2015;2015:1-14. [PMC free article] [PubMed] [Google Scholar]
Gądek-Michalska A, Tadeusz J, Rachwalska P, Bugajski J. Cytokines, prostaglandins and nitric oxide in the regulation of stress-response systems. Pharmacol Rep 2013;65:1655-62. [PubMed] [Google Scholar]
Cleveland Clinic. Blood Brain Barrier. Cleveland Clinic. https://my.clevelandclinic.org/health/body/24931-blood-brain-barrier-bbb
Jang DI, Lee AH, Shin HY, Song HR, Park JH, Kang TB, Lee SR, Yang SH. The Role of Tumor Necrosis Factor Alpha (TNF-α) in Autoimmune Disease and Current TNF-α Inhibitors in Therapeutics. Int J Mol Sci. 2021 Mar 8;22(5):2719. doi: 10.3390/ijms22052719. PMID: 33800290; PMCID: PMC7962638.
Gądek-Michalska A, Tadeusz J, Rachwalska P, Bugajski J. Cytokines, prostaglandins and nitric oxide in the regulation of stress-response systems. Pharmacol Rep 2013;65:1655-62. [PubMed] [Google Scholar]
Ohland CL, Kish L, Bell H, et al. Effects of lactobacillus helveticus on murine behavior are dependent on diet and genotype and correlate with alterations in the gut microbiome. Psychoneuroendocrinol 2013;38:1738-47. [PubMed] [Google Scholar]
Muscatello MRA. Role of negative affects in pathophysiology and clinical expression of irritable bowel syndrome. World J Gastroenterol 2014;20:7570. [PMC free article] [PubMed] [Google Scholar]
Berk M, Williams LJ, Jacka FN, et al. So depression is an inflammatory disease, but where does the inflammation come from? BMC Med 2013;11:200. [PMC free article] [PubMed] [Google Scholar]
Clapp M, Aurora N, Herrera L, Bhatia M, Wilen E, Wakefield S. Gut microbiota's effect on mental health: The gut-brain axis. Clin Pract. 2017 Sep 15;7(4):987. doi: 10.4081/cp.2017.987. PMID: 29071061; PMCID: PMC5641835.
Medical Review Board. What are Stress Hormones and How Do They Impact Your? Body Logic MD. What Are Stress Hormones and How Do They Impact You? | BodyLogicMD
Clapp M, Aurora N, Herrera L, Bhatia M, Wilen E, Wakefield S. Gut microbiota's effect on mental health: The gut-brain axis. Clin Pract. 2017 Sep 15;7(4):987. doi: 10.4081/cp.2017.987. PMID: 29071061; PMCID: PMC5641835.
Mayo Clinc Staff. Yeast infection (vaginal). Mayo Clinic. Yeast infection (vaginal) - Symptoms and causes - Mayo Clinic
Hughes DT, Sperandio V. Inter-kingdom signalling: communication between bacteria and their hosts. Nat Rev Microbiol 2008; 6:111–120. doi: 10.1038/nrmicro1836. [PMC free article] [PubMed] [Google Scholar]
Clapp M, Aurora N, Herrera L, Bhatia M, Wilen E, Wakefield S. Gut microbiota's effect on mental health: The gut-brain axis. Clin Pract. 2017 Sep 15;7(4):987. doi: 10.4081/cp.2017.987. PMID: 29071061; PMCID: PMC5641835.


Facebook
Instagram